Unleashing the Hidden Potential
Reframing Pathology Technology's Role in Australian Healthcare

__INDEX
EXECUTIVE SUMMARY
Pathology testing and technology underpins every aspect of our medical system. It plays a vital role across all facets of healthcare throughout our lives by providing crucial diagnostic information, enabling the selection of proper treatments, aiding in choosing preventive actions, and offering vital data to enhance care management.
Pathology technology is a rapidly evolving area, continuously offering faster, more informative results for patients who have access to these innovations. Faster integration of these innovative technological advances enables the creation of a better, more robust healthcare system that optimises patient-centred care in every instance, regardless of geography, age, and socioeconomic status. Failure to appropriately invest in and fund this innovative technology in a timely fashion impacts the present-day quality of our healthcare system. Our future capability is enhanced by integrating new advancements to upgrade the care we provide to Australians.
As Australia’s population ages and the risk of chronic diseases, cancer, and new infections or pandemics rise, the Australian healthcare system and those around the world are navigating challenges to address the unmet healthcare needs of patients. At the same time, Governments have a responsibility to ensure that healthcare spending remains sustainable while providing equal access to high-quality care.
Our failure to recognise the broader value of pathology technology is resulting in stagnant funding processes and slow adoption of innovative technologies that includes genomics, point of care and digital health. Intangible value, such as the value of knowing and the value of community, are overlooked. There is also inadequate consideration of the cost and realised value that shifts between federal jurisdictions and that of States and Territories. The complexity of these factors results in a lost opportunity to measure the true impact of life-changing technology.
We have an opportunity to invest and provide Australians with timely access to such technology so that Australia does not miss out on direct savings to the healthcare system and the broader social and economic benefits that pathology technology has to offer.
This Health Economic Report shines a light on the true value of pathology technology to health consumers, healthcare providers and to our economy. Providing an evidence base to catalyse the significant change we need to evolve how healthcare is delivered in Australia. The pathology technology revolution affords an opportunity to move from a sickness to a wellness focused healthcare system. We can identify health conditions earlier, initiate treatment sooner and keep people healthy and productive.
While this report highlights just a handful of cases, the underlying technologies address a broad range of conditions, from cancers to mental health, contagions, and antibiotic resistance, as well as in the optimisation of therapeutics for targeted care. There is substantial opportunity to change the way healthcare is delivered, and now is the time to embrace this change.
$7.0 BILLION
in value FORGONE over 2-20 years
CHILDREN WITH SUSPECTED GENETIC CONDITIONS
The high cost of delaying access to innovative genomic tests: Whole exome/genome sequencing.
Next generation sequencing techniques such as whole exome sequencing, whole genome sequencing and rapid whole genome sequencing have revolutionised the diagnosis of rare genetic conditions and continue to rapidly advance in their speed and accuracy. Barriers preventing faster access to these diagnostic technologies has resulted in a loss of over $529.6 million in value over the past 5 years.
Realise the value with a diagnostics expert advisory body.
$15.2 M
in benefits forgone by patients with suspected genetic conditions through better access to support and special education services.
$152.4 M
in benefits forgone by parents of children with suspected genetic conditions through reduced productivity loss, the value of knowing, improved mental wellbeing, increased opportunity for cascade testing and reduced out of pocket costs.
$361.9 M
in benefits forgone by the Government through reduced spending on appointments, tests, treatments, reduced hospital length of stay and mental healthcare services, as well as reduced lives lost.
Having the right test at the right time for known cardiovascular conditions can be life changing
SUSPECTED HEART FAILURE IN THE GP SETTING
Optimising the utilisation of scarce healthcare resources: NT-proBNP.
The delay in providing access via MSAC to NT-proBNP in the GP setting has resulted in an estimated loss of $5.9 billion in value over the past 20 years that could have positively impacted patients, their carers and the Government.
Realise the value with an accelerated funding model
$50.6 M
in benefits forgone by patients with suspected heart failure through reduced productivity loss and reduced out of pocket costs.
$26.2 M
in benefits forgone by friends/family of patients with suspected heart failure through reduced productivity loss.
$5.8 B
in benefits forgone by the Government through a reduction in ED visits and subsequent hospitalisations, reduced echocardiograms and increased government taxation revenue.
Preeclampsia is still considered an immediate risk and danger for 6 weeks after birth, but its impact on my brain and my body would last much longer than that...
PRE-ECLAMPSIA
Unlocking better pregnancy outcomes while reducing healthcare expenditure: the pre-eclampsia (PE) ratio test.
The delay in providing access to the PE ratio test in Australia means the broader value of the test is not being realised, especially when considering that the test was TGA approved in 2011. An estimated loss of $235.3 million in value has been forgone to the Government over the past 12 years as a result of this delayed access.
$235.3 M
in benefits forgone by the Government through a reduced hospital admissions.
OVARIAN CANCER
Improving efficiencies within the healthcare system: HRD test.
The delay in providing access via MSAC to HRD testing for women with newly diagnosed ovarian cancer who are BRCAwt HRD positive has resulted in an estimated loss of $319.1 million in value over the past 2 years that could have positively impacted patients, their carers and the Government.
Realise the value with an accelerated funding model.
$3.7 M
in benefits forgone by patients with BRCAwt HRD+ ovarian cancer in reduced palliative care costs, reduced productivity loss and improved mental health.
$4.4 M
in benefits forgone by friends/family of patients with BRCAwt HRD+ ovarian cancer through reduced informal care and improved mental health.
$310.8 M
in benefits forgone by the Government through avoided deaths and increased tax revenue.
$160.9 K
in benefits forgone by the employers of carers to patients with BRCAwt HRD+ ovarian cancer through reduced presenteeism.
$1.8 BILLION in POTENTIAL value over 10 years
COVID-19
Towards health equity: COVID-19 point of care (POC) test.
The more infectious a disease and the more remote a community, the more valuable POC testing becomes. The provision of POC testing during a global pandemic when the effects of a viral infection were largely unknown enabled the protection of communities who may have otherwise been hard to reach. The implementation of the POC testing program in a swift manner ensured that the true value of the POC test was realised, saving the Government $112 billion over two years.
$337 M- $1.8 B
in benefits gained by the Government through a reduction in COVID-19 infections which prevented the cost of hospitalisations, ICU admissions and medical evacuations.
HEPATITIS C INFECTION (HCV)
Single visit, lasting cure: HCV POC test.
There is a cure available for those diagnosed with HCV. However, it relies on detection of the infection. The value of a cure can be realised with access to the HCV POC test. Access holds the potential to save lives and enhance health outcomes, saving the Government $1.9-6.2 million per year in lives saved. This can also bring us closer to achieving the WHO’s goal of HCV elimination by 2030.
$1.9- $6.2 M
in net benefits gained by the Government through the avoidance of between 9 and 29 HCV deaths as a result of the implementation of the HCV program for one year.
MENINGITIS AND ENCEPHALITIS
Timely diagnostics, tangible savings: the multiplex polymerase chain reaction (PCR) test.
The impact of the multiplex PCR test is far- reaching. Multiplex PCR testing in children with CNS infections enables access to the right treatment at the right time, helping to ensure antibiotics are used appropriately and reducing the risk of death, illness and time spent in hospital. Providing access to multiplex PCR testing could result in the creation of $35.2 million in value over 10 years.
$35.2 M
In potential benefit to the Government through the reduction in hospital length of stay.
MAJOR BLEEDING
Save lives with less blood: POC coagulation test.
The POC coagulation test as part of patient blood management has the potential to enhance healthcare delivery and optimise resources throughout Australia, moving beyond the specific states or hospitals that currently use it. With faster and more precise treatment decisions, the POC test improves patient health outcomes and reduces medical expenditures, resulting in the creation of between $1.4 and $1.8 billion in value over 10 years if adopted nationally.
$1.4-$1.8 B
In potential value to the Government through the reduction in blood product utilisation, hospital length of stay and transfusion administration.
CALL TO ACTION
As stated in the recent Intergenerational Report, “Australia’s ability to meet challenges while seizing future opportunities depends on choices made today”. This report highlights the forgone value and cost to the health economy of delaying access to high-medical value tests and technology. Unlocking the true value of pathology technology for the patients of today will reduce the pressures on the future of healthcare. If a forward-thinking mindset is adopted, Australia can achieve better patient care at a lower total cost with a more robust healthcare system.

- Establish a Diagnostic Advisory Group with stakeholders including healthcare professionals and laboratory service providers, patient advocacy groups, industry bodies, and Government representatives.
- Draw on the Advisory Group for the formation of a National Diagnostics Strategy and Roadmap
- Use the Strategy to guide a range of critical activities such as sovereign supply, stockpile and manufacturing; purposeful R&D and horizon scanning for high-medical value technology; adjudication on accelerated access pathways to fund high-medical value technologies; identification of technology needs for Australia’s key healthcare programs, working with manufacturers to ensure objectives of the programs are met.
- Use the Advisory Group in an ongoing capacity to provide input on future direction of the Strategy.
- In the short term, the Group could identify currently available, but underutilised, high-medical value tests and technology for accelerated pathways through funding and adoption processes.

INTRODUCTION
This report outlines several specific case studies assessing the broader social and economic benefits of early investment into innovative pathology technologies or the impact of delayed investment.
Australia’s healthcare system, and healthcare systems worldwide, are facing challenges in meeting the needs of patients due to the ageing population and the increasing risks of chronic diseases, cancer, and emerging infections or pandemics. At the same time, governments have a responsibility to ensure that healthcare spending remains sustainable while providing equitable access to high-quality care. [8]
Longer, healthier lives can be achieved by preventing or slowing disease progression and facilitating recovery through prompt diagnosis and appropriate clinical management. Pathology technology assists by providing crucial diagnostic and prognostic information, enabling the selection of appropriate treatments, aiding in choosing preventative actions, and offering vital data to improve patient care. [9]
In Australia, over the past three decades, advancements in pathology technology have played a crucial role in driving ongoing enhancements in diagnostic service efficiency and overall healthcare outcomes [10]. During this period, diagnostic services have benefitted from these innovations – transforming laboratories from testing a few hundred patient samples a day to many thousands, reducing the time it takes to run a test, and taking the testing platforms out of the lab to bring them closer to the patient.
Pathology technology continues to experience remarkable advancements, with innovations emerging at an unparalleled rate. These advancements promise enhanced healthcare system efficiencies and positive patient outcomes. Yet these benefits will only be realised if the Australian healthcare system keeps pace with this innovation, acknowledges the value of such technology, and finds a way to deliver these advancements to all Australians equitably.

This report…
Seeks to uncover the unrealised value of pathology technology
This report will…
Showcase 8 pathology technologies and the broader social and economic impacts
The challenge for Australia, and all countries, lies in evaluating the affordability of pathology technology and determining how to gauge the value of these technological advancements. Health Technology Assessment (HTA) is the preferred tool for the Australian Government to assess the value of new technologies. In Australia, entities like the Medical Services Advisory Committee (MSAC) heavily depend on HTA, and MSAC plays a pivotal role in recommending which new medical technologies and services should receive funding for the Australian population. Nonetheless, both MSAC and other state funding organisations tend to have a limited focus, primarily emphasising traditional clinical and health economic aspects. The full range of potential benefits of diagnostic technology beyond these traditional measures – such as providing patients and their families with knowledge or hope – is often overlooked [8]. As a result, we overlook the broader economic value in terms of cost- containment as well as fostering improved health outcomes, ultimately leading to more efficient use of resources.
Australia has been slow to fund the adoption of innovative pathology technology, including genomic, point of care and digital health technologies. Barriers in the funding process delay the investment required for funded access, thus Australia misses out on direct savings to the healthcare system as well as the better patient outcomes, and broader social and economic benefits that pathology technology has to offer.
A paradigm shift is required for MSAC and other state funding bodies to realise the broader value of pathology technology and view it as an investment rather than a cost. We also need an accelerated pathway for funding and adoption of high-value
medical tests and technologies and those that address under-served needs.
BACKGROUND
WHAT IS PATHOLOGY TECHNOLOGY?
Pathology technology drives the provision of high-quality, accessible, and affordable healthcare services. It stands as a cornerstone in modern medicine, offering critical insights that influence patient care decisions every day. Over 70% of medical diagnoses and management decisions rely on pathology test results, including 100% of cancer diagnoses. Early disease detection using this technology aids healthcare provision by informing care decisions, leading to decreased healthcare costs [8].
Pathology technology is at the heart of Australia’s health care system.
It underpins every aspect of medicine, from diagnostic testing and monitoring of chronic diseases to cutting-edge genetic research and blood transfusion technologies.
Every advancement in this field improves our ability to detect diseases earlier, understand them better, and treat them more effectively. For instance, the rise of molecular diagnostics has revolutionised our approach to various cancers, allowing for targeted therapies tailored to individual genetic profiles. Moreover, in an era where infectious diseases can swiftly become global pandemics, rapid and accurate diagnostic tools in pathology become our first line of defence, guiding timely interventions. The true value of pathology technology, therefore, lies not just in its diagnostic precision but in its profound impact on enhancing patient outcomes, reducing resource use, and improving health inequalities.
The future of patient care relies on a healthcare system that is resilient for future populations – technology and patient, family and society outcomes are not separate entities, they are intricately connected components.
RELY ON PATHOLOGY FOR
DIAGNOSIS AND CARE MANAGEMENT
PATHOLOGY
APPLICATIONS
Predict
Predict susceptibility to disease
PREVENT
DIAGNOSE
Diagnose many diseases. For cancer, every case is diagnosed by pathology
DETERMINE
Determine patient prognosis
IDENTIFY
MONITOR
PERSONALISE
INNOVATION IN PATHOLOGY
Faster integration of these innovative technological advances creates a better, more robust healthcare system that optimises patient-centred care in every instance, regardless of geography, age and socioeconomic status.
Earlier in 2023, the World Health Organization (WHO) conducted a horizon scan to identify trends and emerging technologies in health which could be harnessed to improve the health of populations [11]. The report highlighted the importance of adopting a “future-thinking mindset” to ensure equitable and timely access to these new innovations and ranked these technologies into the overall top five most promising areas of innovation. Unsurprisingly, pathology technology featured – directly or indirectly – in all five areas. Timely investment in such technology is crucial for the Australian healthcare system, ensuring it remains adaptive, responsive, and equipped to provide world-class care.
TOP 5 AREAS OF
PROMISING INNOVATION
01
Genomics
Application of genomics for early diagnosis and pre-diagnosis of diseases to guide management and treatment.
Example: Whole genome sequencing to diagnose individuals with rare disease.
02
Vaccines
Better coordinated, more effective systems of vaccine production and global distribution.
Example: Genetic sequencing of the coronavirus to better understand its makeup and develop an mRNA vaccine [190]
03
Low-cost Viral Diagnosis
Rapidly design and construct cost-effective point-of-care diagnostics for viruses.
Example: POC test to diagnose Hepatitis C and provide a cure (see pg. 55) [14]
04
Antimicrobial Drugs
Broad-spectrum antimicrobial drugs that do not cause resistance or tolerance; e.g. adapt their conformation to structural changes or mutations in the target.
Example: Genetic sequencing of infectious organism that cause pneumonia to better understand their makeup and develop drugs which evade their processes or resistance [15]
05
Rapid Remote Diagnostics
Connect people through cell phones, watches and other devices that can provide information on key markers and link health information in real-time to clinicians, people and other (public) health entities, supporting individual health promotion, disease prevention and disease (self) management.
Example: Wearables which conduct ECGs that assist in the diagnosis of cardiovascular disease [16]
Making sure all Australians have access to the latest medicines and medical tests is an Albanese Government priority.
FUNDING INNOVATIVE PATHOLOGY TECHNOLOGY
Australia needs equitable and timely access for the full value of pathology technology to be realised. However, the key to access relies upon changes to our current policies, frameworks, and funding of innovative pathology technology.
Pathology relies on a mix of federal funding from the Medicare Benefits Scheme (MBS), state funding from public hospitals, and clinical trials. State Governments assign funds to Local Health Networks, which in turn distribute them to their respective hospitals. Typically, hospitals have a set budget designated for pathology. [17]
Laboratories typically charge hospitals for pathology tests on a fee-for-service basis. Federal Medicare funding is also fee-for- service according to the MBS item schedule fee. Despite the lower relative cost of testing compared to treatments, pathology has typically been underfunded compared to pharmaceuticals [17]. In addition, coupled with the underfunding of pathology, MBS funding is not directed to the technology supplier but to the laboratory that completes the test. The suppliers thus rely on a trickle down of reimbursement [18]. Technology that falls outside the use pattern of private or public laboratories (such as point of care (POC) testing, or at- home testing) also does not meet the current funding model criteria of ‘fee-for- service’.
Disconnect between state and federal funding of pathology technologies
Australia has a complex and fragmented healthcare system that splits and shares the funding and provision of services between the federal and state governments. The division or roles and responsibilities between levels of Government results in “cost and blame shifting” leading to gaps in services [19] and also budget silos that undermine efficiency [20].
The current financing structure reflects a complex and fragmented health care system that struggles to provide effective patient- centred care. Patients experience a lack of access to care, inequities of access depending on where they live, and delays in access, all of which could result in poorer health outcomes [20].
MSAC pathways to reimbursement are long and complex
The MSAC process is often described as a rate- limiting step to accessing innovative technologies and subsequent treatment. The MSAC process, including the data generation and modelling requirements for HTA, is long and complex, which does not match the evolution of innovation and clinical practice. [21, 22]
Currently, the funding application process has many redundancies, requires local data generation, and can be a costly and time- consuming process, thus access to new and innovative technologies is slow. This is evidenced by the fact that Medicare’s coverage is limited compared to the number of tests available on the Australian Register of Therapeutic Goods (ARTG) in Australia. [17]
Reimbursement or procurement decisions do not recognise the broader value of pathology technology
State procurement and HTA processes currently undervalue the broader benefits of pathology technology, particularly the value to other parts of the healthcare system.
In order to harness the innovation pipeline, the evaluation process needs to consider and reliably measure the many ways that pathology technology can create value beyond the traditional clinical and safety outcomes. This includes but is not limited to a broad array of patient-centric values such as increased knowledge and hope for a cure, as well as broader healthcare system values including resource optimisation and hospital savings. [17]
As Australia and the world continue to develop novel pathology technologies, we require flexibility and adaptability to ensure they are valued appropriately. At present, there is unrealised value in innovative pathology technology that Australia is failing to tap into.
THE UNREALISED VALUE OF PATHOLOGY TECHNOLOGY
As the pathology technology landscape is vast, dynamic, and continually evolving, the way we assess the funding of such technology must similarly adapt. Relying solely on traditional parameters of clinical and safety outcomes, limited only to immediate returns or a narrow scope of value, is inadequate. The broader value of such technology often lies beyond these primary outcomes, having deeper and sometimes intangible effects on society, including families, carers, employers, and various other sectors of state and federal Government.
To truly understand and appreciate the multifaceted value of pathology technology, funding assessments must consider both the indirect and direct impacts, taking into account the various stakeholders it can benefit. For instance, pathology technologies might not only diagnose diseases, but allow treatment to be identified, enhance workforce productivity, reduce immediate and long-term healthcare costs, and improve general wellbeing for both the patient, their families, and communities.
To demonstrate the elements of value, this report includes a Value Fountain, drawing on previous work [23], which highlights the numerous elements of value that may be overlooked or underappreciated in technology assessments. If Australia is to futureproof our healthcare system and continue to support the development of innovative pathology technologies, a paradigm shift in our perception of value is required.
Neglecting these broader benefits in a Health Technology Assessment (HTA), risks underestimating or undervaluing the transformative potential of innovative technologies, possibly stalling their future development, while denying access to patients. As such, a more holistic or ‘whole of Government’ approach to valuing technology is needed. It will ensure that we are not solely focussing on the health budget and limited definition of the value technology can provide, but that we leverage the expansive potential technology can bring to our society.
THE VALUES
RESOURCE OPTIMISATION
Fast and accurate diagnoses, minimises the need for unnecessary tests, treatments, and hospital stays, facilitating the strategic use of resources.
Knowledge Dissemination
Results of a test can be used in research and for future generations of patients.
Community
Stopping the spread of disease creates a safe and vibrant society.
Efficency
Improved patient outcomes while reducing direct and indirect costs.
Personalisation
Using test results to tailor individualised treatment options, enhancing patient outcomes through personalised care.
Responsiveness
Rapid response to personal or public health crises and helps to control outbreaks, informs the need for vaccines and strengthens the health system.
Prevention
Early identification of at-risk patients improves patient outcomes and reduces cost.
Equity
Redistribution of health from equal access to essential services and resources.
LIFE
The intrinsic and inherent worth of every person
Hope
Benefits derived by a patient (or a patient’s family or carers) from knowing the results of a test that may provide a treatment or a cure in the future.
KNOWING
Harms avoided and benefits derived by a patient (or a patient’s family or carers) from knowing the results of a test or obtaining a diagnosis.
CURE
Elimination of a disease for individuals and the broader society.
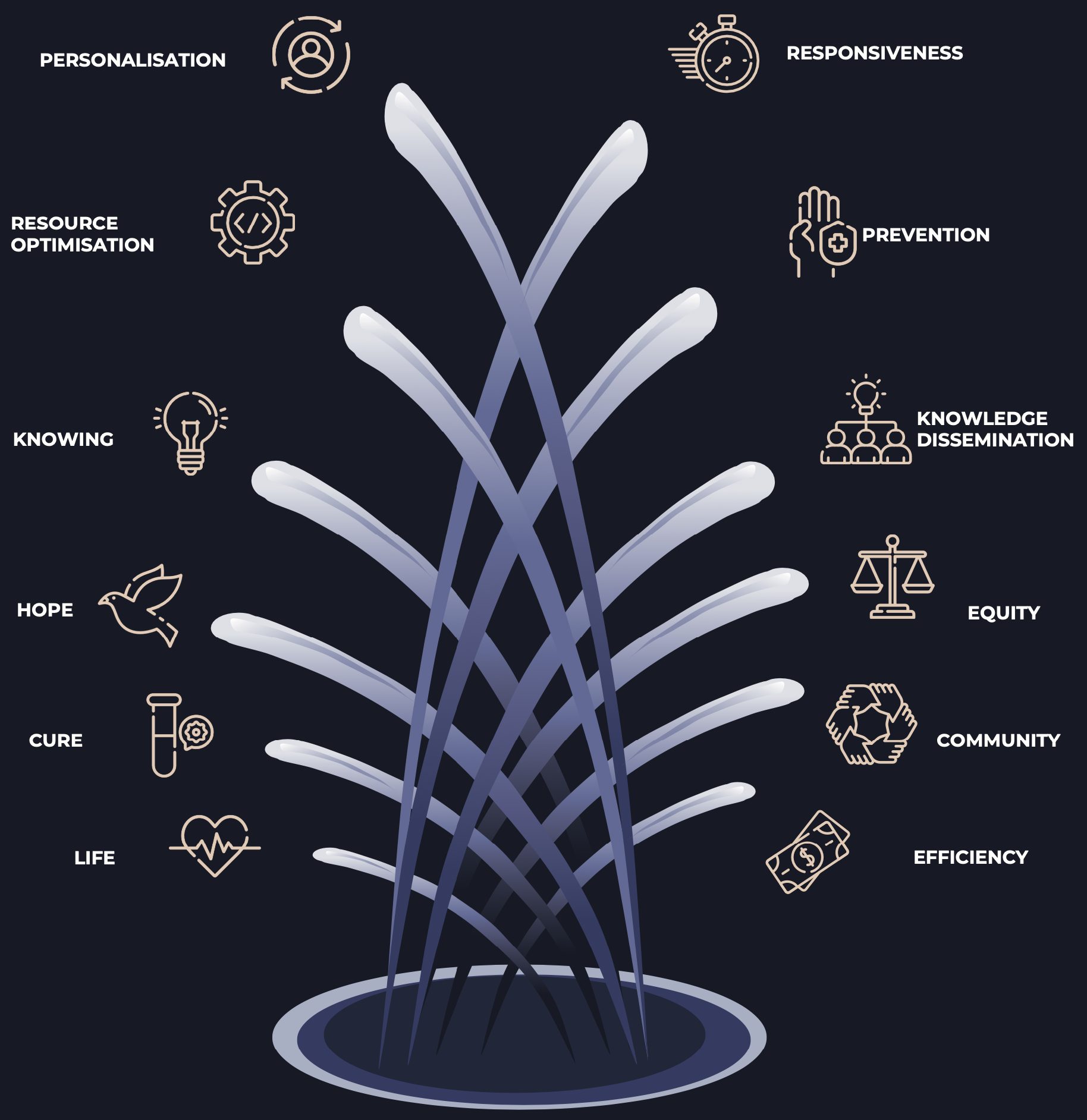
THE VALUE FOUNTAIN
PATHOLOGY TECHNOLOGY -> DIRECT AND INDIRECT IMPACT -> VALUE
Diagnostics was the unsung hero in my story. It was testing that guided my diagnosis, my treatment and survival. Early Detection became my lifeline and without that, I wouldn’t be here and about to become a dad.
OVERVIEW
OF CASE STUDIES
The innovation provided by pathology technology can be transformative, and timely access to such technologies can bring a vast array of benefits to the Government, patients, families, and the community. Value that could have been provided by NT -proBNP and the PE ratio test are still yet to be realised. Value brought by the HRD test as well as the WES and rWGS was delayed by many years due to the long HTA process.
TOTAL VALUE FOREGONE
PERSONALISATION |
RESOURCE OPTIMISTION |
KNOWING |
HOPE |
CURE |
LIFE |
RESPONSIVENESS |
PREVENTION |
KNOWLEDGE DISSEMINATION |
EQUITY |
COMMUNITY |
EFFICIENCY |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heart Failure | ||||||||||||
| Pre-Eclampsia | ||||||||||||
| Ovarian Cancer | ||||||||||||
| Genetic Conditions |
TOTAL VALUE GAINED
PERSONALISATION |
RESOURCE OPTIMISTION |
KNOWING |
HOPE |
CURE |
LIFE |
RESPONSIVENESS |
PREVENTION |
KNOWLEDGE DISSEMINATION |
EQUITY |
COMMUNITY |
EFFICIENCY |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COVID | ||||||||||||
| Hepatitis C |
TOTAL POTENTIAL VALUE
PERSONALISATION |
RESOURCE OPTIMISTION |
KNOWING |
HOPE |
CURE |
LIFE |
RESPONSIVENESS |
PREVENTION |
KNOWLEDGE DISSEMINATION |
EQUITY |
COMMUNITY |
EFFICIENCY |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood Management | ||||||||||||
| Bacterial/Viral Infections |

REALISE THE VALUE OF PATHOLOGY TECHNOLOGY
To continue to realise the broad value of pathology technology including those tests highlighted in this report, Australia should adopt a model for pathology technology that changes our core perspective.
The overarching vision is a healthier Australia firmly based on a Wellness Economy. With this foundation, future policy and funding decisions move us incrementally but purposefully towards this outcome. This vision firmly supports and is a key contributor to the Treasurer’s Wellbeing Framework.
A multi-disciplinary advisory group that includes healthcare professionals and service providers, patient advocacy groups, industry bodies, and Government representatives. The group would advise on IVD-related health policy and funding, including the creation of a National Diagnostics Strategy and Roadmap, and adjudicate on high-medical value IVDs. They could also conduct periodic horizon scanning activities and potentially assist in identifying high-value Australian IVD innovations for accelerated local commercialisation initiatives to boost our sovereign capacity and support high-tech manufacturing. This group could also form the link between our major health strategies, such as the recently announced Australian Cancer Plan, and service providers, ensuring technology suppliers can develop and supply devices required to achieve our national healthcare goals.
Patient-Centred Strategic Elements to Support National Health Goals
Under the guidance of a Diagnostics Advisory Group, we could ensure our National Diagnostics Strategy and Roadmap (NDSR) had a strong patient-centric focus, encompassing the full Value Fountain as outlined in this report. Pathology technology is a keystone of our healthcare system – impacting almost every element of healthcare from diagnosis to prognosis. We must address the existing barriers to access if we are to secure the success of any other national healthcare priority. A brief overview of the priorities and key asks on the Department of Health and Aged Care demonstrate the clear need for a nationally coordinated approach that draws on the subject matter expertise of those currently supplying, delivering, and safeguarding our healthcare services.
Recent reports from Government, Patient Advocacy Groups, and Peak Bodies:
1 The Diagnostic Technology Sovereign Capability & Resilience National Action Plan identified the role of a NDSR as well as a legislated EAG to drive sustainable sovereign capability in key areas of the IVD sector.
2 The Intergenerational report acknowledges ““escalating health pressures, it will be important to ensure that the health system provides value for money. This requires a health system that innovates and prioritises funding a patient- centred and sustainable Australian healthcare system that delivers the best outcomes for communities This will require funding arrangements that continue to effectively invest in preventative health and evidence-based health care spending.”
3 The Department of Health and Aged Care Capability Review identified a critical challenge to “increasing focus on preventative healthcare”, the “need to address inequities between different population groups” and “the need to improve the sustainability of the health system”. That same report acknowledged the need for the Department to be “on the front foot in terms of opportunities in the rise of personalised medicine, mRNA technology, digital technologies, the promise of artificial intelligence and the revolution in genomics” – all of which can be served through a NDSR and Diagnostic Advisory Group.
4 Rare Cancer’s Rarefication: Personalised medicine in the genomic revolution calls to ”progress a coordinated national genomics strategy, as well as the need to adequately prioritise research into genomic studies and precision oncology”. The same report calls for “a new pathway in our Health Technology Assessments that encompasses broader value measures”.
5 CSIROs Strengthening Australia’s Pandemic Preparedness calls to “develop a diagnostics deployment strategy for scaling POC testing applications,” implement a diagnostics development program aimed at small and medium sized enterprises”, and to “Strengthen translational science to help bridge the gap between research, industry and the health system”.
6 ATSE Tech-Readiness Health Report highlighted technology as a solution to improve equity of access to healthcare and called on Government to support “investment in improving pathways to commercialisation for Australian-developed medical technology”.
7 Cancer Australia Australian Cancer Plan highlights the need for preventative and early detection measures that rely on a streamlined process of funding evolution in genetic tests and technology that underpin these goals.
THE PATH
FORWARD
In today’s dynamic landscape of healthcare innovation, pathology technology stands as a beacon of promise, offering transformative potential for improving patient outcomes and advancing healthcare in Australia. It is a pivotal moment for the nation, where embracing this often under-valued facet of healthcare can take Australia from a nation primarily treating chronic diseases to a proactive healthcare society that pre-empts and manages illnesses before they take a firm grip. The discourse in this report presents an irrefutable case for the Australian federal Government to recognise the invaluable contribution of pathology technology and cultivate a comprehensive perspective.
Pathology technology cannot be confined as a budgetary line item in Australia’s healthcare system. When harnessed with a clear purpose, it forms an essential cornerstone of our healthcare infrastructure with broad-reaching effect. Its significance is being recognised globally; the World Health Organization acknowledges pathology technology as one of the five most promising areas of innovation, and the recent World Health Assembly (WHA) is urging Member States to elevate the role of diagnostics in their healthcare approach.
Timely investment in pathology technology provides gains in healthcare system efficiency, advances in disease prevention, and optimisation of responses and resource management. This value transcends obvious fiscal gains; benefiting the Government, patients, families, and society at large. Timely investments in this space go beyond the pioneering of healthcare solutions; it’s an opportunity to fortify Australia’s leadership position in an ever-evolving medical field.
Our current reimbursement pathways – complex and outmoded – struggle to keep pace with technological advancement. Fragmented funding structures, distributed across federal and state governments, foster inefficiency and impede the timely provision of innovative pathology technologies. Delayed access to these technologies has imposed a high cost that extends beyond dollars and cents. This report demonstrates over $7.0 billion has been forfeited due to delays in providing funded access to high-medical value technologies.
Consider, for instance, the delayed introduction of GP-requested NT-proBNP for suspected heart failure or the PE ratio test for pre-eclampsia. A staggering $5.9 billion could have been preserved by averting ED visits. Furthermore, timely access to the PE ratio test could have spared the Government another $235 million in reduced hospital admissions. Notably, the delay in providing access to WES/WGS meant that 1,068 additional children were left without a diagnosis, missing out on the vital support they and their families needed.
The Government’s potential benefits from acknowledging the value of pathology technology are substantial. Programs such as COVID-19 and HCV POC testing exemplify the immense benefits of early detection and patient-centred testing. These advantages translate to a substantial value of at least $1.8 billion, potentially saving countless lives. For instance, compared to conventional testing methods, the HCV program using POC testing saved an estimated 8 lives within a single year.
Embracing the broader spectrum of innovative pathology technology, including the Multiplex Assay for CNS infections and the POC coagulation test, could yield over $1.8 million in avoided hospital costs over the coming decade. Pathology technology holds the key to superior resource utilisation, heightened healthcare efficiency, and improved patient health outcomes. The development of the “Value Fountain” concept underscores the untapped potential that must be harnessed when considering investments in this field.
The recent Intergenerational Report underscores the mounting cost pressures on healthcare expenditure, driven by an ageing population, increased rates of chronic disease, and the escalating demand for high-quality healthcare services. This juncture represents an opportune moment for Australia to pioneer a nationally consistent framework for comprehensively assessing technology’s value in healthcare, including pathology technology.
Australia urgently requires a more comprehensive and inclusive approach that encapsulates the total value of pathology technology to patients, their families and to Government (both in terms of costs and lost productivity). In addition to HTA reform, the provision of an accelerated access program for high-medical value and promising innovative technologies is essential to curtail access delays.
A natural progression from this recognition is the development of an overarching Diagnostics Advisory Group to devise a pathology technology strategy for Australia – a NDSR. This comprehensive strategy should encompass a National Genomic Strategy (to replace the guidance that expired in 2021), digital health initiatives, including the strategic use of AI, POC testing, and a roadmap for innovative technology development, as recommended in the ADAPT Diagnostics Report [189].
As Australia stands at the threshold of transformative change in healthcare, pathology technology offers a wealth of opportunity. Through visionary leadership, innovative strategies, and judicious investments, we can unlock pathology technology’s full spectrum of value, securing a brighter and healthier future for Australia.

References
- The Royal College of Pathologists of Australasia. Pathology - the facts 2021 31/08/23. Available from: https://www.rcpa.edu.au/Library/Fact- Sheets/Pathology-The-Facts.
- England N. National Pathology Programme Digital First: Clinical Transformation through Pathology Innovation2014 31/08/23. Available from: https://www.england.nhs.uk/2014/02/n pp-digital-first/.
Economics TCfI. The economic value of pathology: achieving better health, and a better use of health resources. thecie.com.au: The CIE; 2016 April 2016.
- Solutions DCfH. The Future of Diagnostics Technology driven personalised and preventive healthcare in Europe2022 31/08/23. Available from: https://www.deloitte.com/global/en/Industries/life-sciences-health- care/research/gx-future-of- diagnostics.html.
- World Health Organization. Strengthening diagnostics capacity2023 09/11/23. Available from: https://www.who.int/news/item/27-05-2023-seventy-sixth-world-health-assembly---daily-update--27-may-2023.
Roche Diagnostics Australia. Analysis of applications considered by the MSAC 2016-2022.
- MP THMB. Improve use of medicines and medical tests with $26 million in grants2023 31/10/23. Available from: https://www.health.gov.au/ministers/th e-hon-mark-butler- mp/media/improve-use-of-medicines- and-medical-tests-with-26-million-in- grants.
Wurcel V, Cicchetti A, Garrison L, Kip Michelle MA, Koffijberg H, Kolbe A, et al. The Value of Diagnostic Information in Personalised Healthcare: A Comprehensive Concept to Facilitate Bringing This Technology into Healthcare Systems. Public Health Genomics. 2019;22(1- 2):8-15.
OECD. Health at a Glance: Europe 2022 2022 [cited 2023 3 October].
- Pathology Technology Australia. Intergenerational Report 2023 – Opportunities for Pathology Technology and an Australian Wellness Economy 2023 [cited 2023 3 October]. Available from: https://pathologytechnology.org.au/i ntergnerationalreport2023/
- Organization WH. Emerging technologies and scientific innovations: a global public health perspective 2023 16/08/23:[25 p.].
Lunke S, Bouffler SE, Patel CV, Sandaradura SA, Wilson M, Pinner J, et al. Integrated multi-omics for rapid rare disease diagnosis on a national scale. Nature Medicine. 2023;29(7):1681-91.
Tan TY, Dillon OJ, Stark Z, Schofield D, Alam K, Shrestha R, et al. Diagnostic Impact and Cost-effectiveness of Whole-Exome Sequencing for Ambulant Children With Suspected Monogenic Conditions. JAMA Pediatrics. 2017;171(9):855-62.
Shih STF, Cheng Q, Carson J, Valerio H, Sheehan Y, Gray RT, et al. Optimizing point-of-care testing strategies for diagnosis and treatment of hepatitis C virus infection in Australia: a model-based cost-effectiveness analysis. The Lancet Regional Health – Western Pacific.
Quinton LJ, Walkey AJ, Mizgerd JP. Integrative Physiology of Pneumonia. Physiol Rev. 2018;98(3):1417-64.
Bayoumy K, Gaber M, Elshafeey A, Mhaimeed O, Dineen EH, Marvel FA, et al. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nature Reviews Cardiology. 2021;18(8):581-99.
Rare Cancers Australia. Australian Cancer Genomics Landscape Assessment; Step 1, toward building an Australian Cancer Futures Framework – National Oncology Alliance rarecancers.org.au; 2020.
Pathology Technology Australia. Pathology Technology Australia’s Submission to the House of Representatives Standing Committee on Health, Aged Care and Sport Inquiry into approval process for new drugs and novel medical technologies in Australia. aph.gov.au: Australian Parliament House 2021.
McPake B, Mahal A. Addressing the Needs of an Aging Population in the Health System: The Australian Case. Health Syst Reform. 2017;3(3):236-47.
Angeles MR, Crosland P, Hensher M. Challenges for Medicare and universal health care in Australia since 2000. Medical Journal of Australia. 2023;218(7):322-9.
- InGeNA. Realising the full potential of genomics to personalise healthcare 2022 [cited 2023 22 September]. Available from: https://ingena.org.au/patient-access/.
- Parliament of Australia. 7. The Medical Services Advisory Committee n.d. [cited 2023 22 September]. Available from: https://www.aph.gov.au/Parliamentary _Business/Committees/House/Health_ Aged_Care_and_Sport/Newdrugs/Rep ort/section?id=committees%2freportre p%2f024755%2f77599.
Neumann PJ, Garrison LP, Willke RJ. The History and Future of the “ISPOR Value Flower”: Addressing Limitations of Conventional Cost-Effectiveness Analysis. Value in Health. 2022;25(4):558-65.
Heart Foundation. What is heart failure? heartfoundation.org.au: Heart Foundation 2023 [Available from:
Australian Institute of Health and Welfare. Heart, stroke and vascular disease: Australian facts. Canberra: AIHW; 2023.
Atherton JJ, Sindone A, De Pasquale CG, Driscoll A, MacDonald PS, Hopper I, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Guidelines for the Prevention, Detection, and Management of Heart Failure in Australia 2018. Heart, Lung and Circulation. 2018;27(10):1123-208.
Roche. Heart failure:The hidden costs of late diagnosis. 2020.
Rosenberg J, Schou M, Gustafsson F, Badskjær J, Hildebrandt P. Prognostic threshold levels of NT- proBNP testing in primary care. European Heart Journal. 2008;30(1):66-73.
Audehm R, Neville A, Piazza P, Haikerwal D, Sindone A, Parsons R, et al. Healthcare services use by patients with heart failure in Australia: Findings from the SHAPE study. Australian Journal for General Practitioners. 2022;51:713-20.
Burri E, Hochholzer K, Arenja N, Martin-Braschler H, Kaestner L, Gekeler H, et al. B-type natriuretic peptide in the evaluation and management of dyspnoea in primary care. J Intern Med. 2012;272(5):504-13.
Scott MA, Price CP, Cowie MR, Buxton MJ, Scott M. Cost- consequences analysis of natriuretic peptide assays to refute symptomatic heart failure in primary care. Br J Cardiol. 2008;15(4):199-204.
Australian Bureau of Statistics.
- National Health Service. Health A to Z: Echocardiogram nhs.uk: NHS; 2022 Available from: https://www.nhs.uk/conditions/echoc ardiogram/.
Clark RA, Eckert KA, Stewart S, Phillips SM, Yallop JJ, Tonkin AM, et al. Rural and urban differentials in primary care management of chronic heart failure: new data from the CASE study. Med J Aust. 2007;186(9):441-5.
Walsh WF, Kangaharan N. Cardiac care for Indigenous Australians: practical considerations from a clinical perspective. Med J Aust. 2017;207(1):40- 5.
Chan Y-K, Tuttle C, Ball J, Teng T-HK, Ahamed Y, Carrington MJ, et al. Current and projected burden of heart failure in the Australian adult population: a substantive but still ill- defined major health issue. BMC Health Services Research. 2016;16(1):501.
Sahle BW, Owen AJ, Mutowo MP, Krum H, Reid CM. Prevalence of heart failure in Australia: a systematic review. BMC Cardiovascular Disorders. 2016;16(1):32.
AlHabeeb W. Heart failure disease management program: A review. Medicine (Baltimore). 2022;101(31):e29805.
Use of B-Type Natriuretic Peptide (BNP) and N-Terminal proBNP (NT- proBNP) as Diagnostic Tests in Adults With Suspected Heart Failure: A Health Technology Assessment. Ont Health Technol Assess Ser. 2021;21(2):1-125.
Fonseca C, Bettencourt P, Brito D, Febra H, Pereira Á, Genovez V, et al. NT- proBNP for heart failure diagnosis in Primary Care: Costs or savings? A budget impact study. Revista Portuguesa de Cardiologia. 2022;41(3):183-93.
Roalfe AK, Lay-Flurrie SL, Ordóñez- Mena JM, Goyder CR, Jones NR, Hobbs FDR, et al. Long term trends in natriuretic peptide testing for heart failure in UK primary care: a cohort study. Eur Heart J. 2021;43(9):881-91.
National Institute for Health and Care Excellence. Chronic Heart Failure: Management of chronic heart failure in adults in primary and secondary care (update). nice.org.uk; 2018.
Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74(11):1893-8.
Chami J, Fleming S, Taylor CJ, Bankhead CR, James T, Shine B, et al. Point-of-care NT-proBNP monitoring for heart failure: observational feasibility study in primary care. BJGP Open. 2022;6(3).
- National Institute for Health and Care Excellence. Chronic heart failure in adults: diagnosis and management2018 01/09/23. Available from: https://www.nice.org.uk/guidance/ng 106.
Therapeutic Goods Administration. Public Summary2012 20/09/23.
Barratt AL, Li Y, Gooroovadoo I, Todd A, Dou Y, McAlister S, et al. Environmental impact of cardiovascular healthcare. Open Heart. 2023;10(1).
Australian Bureau of Statistics. Income and work: census abs.gov.au: ABS; 2021 [updated 29/08/23.
- Natasha Cassidy SP. The rising share of part-time employment 2017. Available from: https://www.rba.gov.au/publications/ bulletin/2017/sep/3.html.
Liew D, Audehm RG, Haikerwal D, Piazza P, Neville AM, Lim K, et al. Epidemiology of heart failure: Study of Heart failure in the Australian Primary carE setting (SHAPE). ESC Heart Failure. 2020;7(6):3871-80.
- Australian Institute of Health and Welfare. Emergency department care 2021-22 data tables In: AIHW, editor. aihw.gov.au2022.
Australian Institute of Health and Welfare. Medicare-subsidised GP, allied health and specialist health care across local areas: 2021–22. Canberra: AIHW; 2022.
- Australian Government Department of Health and Aged Care. Risk of pre- eclampsia health.gov.au: Australian Government 2021 [Available from https://www.health.gov.au/resources/ pregnancy-care-guidelines/part-d- clinical-assessments/risk-of-pre- eclampsia: ).
Levine RJ, Maynard SE, Qian C, Lim K- H, England LJ, Yu KF, et al. Circulating Angiogenic Factors and the Risk of Preeclampsia. New England Journal of Medicine. 2004;350(7):672-83
Australian Institute of Health and Welfare. Aboriginal and Torres Strait Islander mothers and babies aihw.gov.au: AIHW; 2023.
National Institiute for Health and Care Excellence. PLGF-based testing to help diagnose suspected preterm pre- eclampsia. nice.org.uk: NICE; 2022.
Vatish M, Strunz-McKendry T, Hund M, Allegranza D, Wolf C, Smare C. sFlt- 1/PlGF ratio test for pre-eclampsia: an economic assessment for the UK. Ultrasound Obstet Gynecol. 2016;48(6):765-71.
Delahaije DH, Smits LJ, van Kuijk SM, Peeters LL, Duvekot JJ, Ganzevoort W, et al. Care-as-usual provided to formerly preeclamptic women in the Netherlands in the next pregnancy: health care consumption, costs and maternal and child outcome. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2014;179:240-5.
Liu A, Wen SW, Bottomley J, Walker MC, Smith G. Utilization of health care services of pregnant women complicated by preeclampsia in Ontario. Hypertension in pregnancy. 2009;28(1):76-84.
Pant V, Yadav BK, Sharma J. A cross sectional study to assess the sFlt-1:PlGF ratio in pregnant women with and without preeclampsia. BMC Pregnancy Childbirth. 2019;19(1):266.
Frusca T, Gervasi MT, Paolini D, Dionisi M, Ferre F, Cetin I. Budget impact analysis of sFlt-1/PlGF ratio as prediction test in Italian women with suspected preeclampsia. J Matern Fetal Neonatal Med. 2017;30(18):2166- 73.
Ohkuchi A, Masuyama H, Yamamoto T, Kikuchi T, Taguchi N, Wolf C, et al. Economic evaluation of the sFlt- 1/PlGF ratio for the short-term prediction of preeclampsia in a Japanese cohort of the PROGNOSIS Asia study. Hypertension Research. 2021;44(7):822-9.
Schlembach D, Hund M, Schroer A, Wolf C. Economic assessment of the use of the sFlt-1/PlGF ratio test to predict preeclampsia in Germany. BMC Health Serv Res. 2018;18(1):603.
- MSAC application 1706. Angiogenic and anti-angiogenic markers for identification and management of preeclampsia [cited 2023 September 14]. Available from: http://www.msac.gov.au/internet/msa c/publishing.nsf/Content/D0768A6F7 597A41ECA2587A5001885C9/$File/170 6%20Redacted%20Application%20Fo rm.pdf.
- Australian Bureau of Statistics. Births, Australia 2022 [cited 2023 September 14]. Available from: https://www.abs.gov.au/statistics/peo ple/population/births-australia/latest- release.
Australian Department of Health and Welfare. Australia's mothers and babies. Canberra: AIHW; 2023.
Boban S, Downs J, Codde J, Cohen PA, Bulsara C. Women Diagnosed with Ovarian Cancer: Patient and Carer Experiences and Perspectives. Patient Relat Outcome Meas. 2021;12:33-43.
Tan JH, Sharpe L, Russell H. The impact of ovarian cancer on individuals and their caregivers: A qualitative analysis. Psychooncology. 2021;30(2):212-20.
- Cancer Council NSW. Ovarian cancer symptoms 2023 [cited 2023 11 October]. Available from: https://www.cancercouncil.com.au/ov arian-cancer/symptoms/.
Australian Department of Health and Welfare. Cancer incidence projections, Australia 2011 to 2020. Canberra: AIHW; 2012.
Australian Department of Health and Welfare. Cancer incidence projections, Australia 2011 to 2020. Canberra: AIHW; 2012.
Australian Department of Health and Welfare. Cancer in Australia 2021. Canberra: AIHW; 2021.
O'Connor MJ. Targeting the DNA Damage Response in Cancer. Mol Cell. 2015;60(4):547-60.
Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. Jama. 2017;317(23):2402-16.
Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi24-32.
Ray-Coquard I, Leary A, Pignata S, Cropet C, González-Martín A, Marth C, et al. Olaparib plus bevacizumab first- line maintenance in ovarian cancer: final overall survival results from the PAOLA-1/ENGOT-ov25 trial. Annals of Oncology. 2023;34(8):681-92.
Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med. 2019;381(25):2416-28.
Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557-88.
Yabroff KR, Kim Y. Time costs associated with informal caregiving for cancer survivors. Cancer. 2009;115(S18):4362-73.
Angioli R, Capriglione S, Aloisi A, Miranda A, de Cicco Nardone C, Terranova C, et al. Economic Impact Among Family Caregivers of Patients With Advanced Ovarian Cancer. Int J Gynecol Cancer. 2015;25(8):1541-6.
Kardamanidis K, Lim K, Da Cunha C, Taylor LK, Jorm LR. Hospital costs of older people in New South Wales in the last year of life. Medical Journal of Australia. 2007;187(7):383-6.
- Australian Bureau of Statistics. Consumer Price Index, Australia; Jun- quarter-2023, Canberra: ABS; [Available from: https://www.abs.gov.au/statistics/eco nomy/price-indexes-and- inflation/consumer-price-index- australia/latest-release.
- Australian Bureau of Statistics. Employee Earnings and Hours, Australia, 2021 May Canberra: ABS; 2021 [Available from: https://www.abs.gov.au/statistics/labo ur/earnings-and-working- conditions/employee-earnings-and- hours-australia/latest-release.
- Australian Bureau of Statistics. Income and work: Census Canberra: ABS; 2021 [Available from: https://www.abs.gov.au/statistics/labo ur/earnings-and-working- conditions/income-and-work- census/latest-release
de Boer AG, Torp S, Popa A, Horsboel T, Zadnik V, Rottenberg Y, et al. Long- term work retention after treatment for cancer: a systematic review and meta-analysis. J Cancer Surviv. 2020;14(2):135-50.
Office of Best Practice Regulation. Best Practice Regulation Guidance Note Value of statistical life. In: Department of the Prime Minister and Cabinet, editor. Canberra: Office of Best Practice Regulation,; 2021.
Office of Impact Analysis. Value of statistical life. In: Department of the Prime Minister and Cabinet, editor. Canberra: Office of Impact Analysis; 2023.
- Australian Bureau of Statistics. Life tables; 2019-2021 Canberra: ABS; 2021 [Available from: https://www.abs.gov.au/statistics/peo ple/population/life-tables/latest- release
Cassidy N, Parsons S. The rising share of part-time employment. RBA Bulletin, September. 2017:19-26.
Ugalde A, Krishnasamy M, Schofield P. Development of an instrument to measure self-efficacy in caregivers of people with advanced cancer. Psychooncology. 2013;22(6):1428-34.
Australian Institute of Health and Welfare. Australian Burden of Disease Study: Methods and supplementary material 2018. Canberra: Australian Institute of Health and Welfare; 2018.
- Australian Bureau of Statistics. National Study of Mental Health and Wellbeing 2020-2022 Canberra: ABS; 2023 [Available from: https://www.abs.gov.au/statistics/healt h/mental-health/national-study- mental-health-and-wellbeing/latest- release.
Reid F, Bhatla N, Oza AM, Blank SV, Cohen R, Adams T, et al. The World Ovarian Cancer Coalition Every Woman Study: identifying challenges and opportunities to improve survival and quality of life. International Journal of Gynecologic Cancer. 2021;31(2):238- 44.
Yang WFZ, Lee RZY, Kuparasundram S, Tan T, Chan YH, Griva K, et al. Cancer caregivers unmet needs and emotional states across cancer treatment phases. PLOS ONE. 2021;16(8):e0255901.
Simon C, P. F. What Causes Genetic Disorders? InnovAiT. 2008;1(8):544-553.
Bauskis A, Strange C, Molster C, Fisher C. The diagnostic odyssey: insights from parents of children living with an undiagnosed condition. Orphanet J Rare Dis. 2022;17(1):233.
Foundation RCH. The Impact of Giving 2020.
Wojcik MH, Stadelmaier R, Heinke D, Holm IA, Tan WH, Agrawal PB. The Unrecognized Mortality Burden of Genetic Disorders in Infancy. Am J Public Health. 2021;111(S2):S156-s62.
Owen MJ, Wright MS, Batalov S, Kwon Y, Ding Y, Chau KK, et al. Reclassification of the Etiology of Infant Mortality With Whole-Genome Sequencing. JAMA Netw Open. 2023;6(2):e2254069.
Zurynski Y, Deverell M, Dalkeith T, Johnson S, Christodoulou J, Leonard H, et al. Australian children living with rare diseases: experiences of diagnosis and perceived consequences of diagnostic delays. Orphanet J Rare Dis. 2017;12(1):68.
House of Representatives Standing Committee on Health ACaS. The New Frontier - Delivering better health for all Australians: Inquiry into approval processes for new drugs and novel medical technologies in Australia. Parliament of the Commonwealth of Australia.; 2021.
Tan TY, Dillon OJ, Stark Z, Schofield D, Alam K, Shrestha R, et al. Diagnostic Impact and Cost-effectiveness of Whole-Exome Sequencing for Ambulant Children With Suspected Monogenic Conditions. JAMA Pediatr. 2017;171(9):855-62.
Farnaes L, Hildreth A, Sweeney NM, Clark MM, Chowdhury S, Nahas S, et al. Rapid whole-genome sequencing decreases infant morbidity and cost of hospitalization. NPJ Genom Med. 2018;3:10.
Baker EK, Arora S, Amor DJ, Date P, Cross M, O’Brien J, et al. The Cost of Raising Individuals with Fragile X or Chromosome 15 Imprinting Disorders in Australia. Journal of Autism and Developmental Disorders. 2023;53(4):1682-92.
- Weiner J, Sharma J, Lantos J, 107. Genetics B. What is Whole Exome Sequencing? : Baylor Genetics 2023 [Available from: https://www.baylorgenetics.com/new s/what-is-whole-exome-sequencing/.
- Roche. WES vs. WGS vs. Custom Panels 2023 [Available from: https://sequencing.roche.com/us/en/ar ticle-listing/wes-wgs-custom.html.
- Department of Health and Aged Care. Medicare Benefits Schedule health.gov.au2023 [Available from: https://www9.health.gov.au/mbs/searc h.cfm?q=73358&Submit=&sopt=S].
Stark Z, Lunke S, Brett GR, Tan NB, Stapleton R, Kumble S, et al. Meeting the challenges of implementing rapid genomic testing in acute pediatric care. Genetics in Medicine. 2018;20(12):1554-63.
Goranitis I, Wu Y, Lunke S, White SM, Tan TY, Yeung A, et al. Is faster better? An economic evaluation of rapid and ultra-rapid genomic testing in critically ill infants and children. Genetics in Medicine. 2022;24(5):1037-44.
Lunke S, Eggers S, Wilson M, Patel C, Barnett CP, Pinner J, et al. Feasibility of Ultra-Rapid Exome Sequencing in Critically Ill Infants and Children With Suspected Monogenic Conditions in the Australian Public Health Care System. Jama. 2020;323(24):2503-11.
Clark MM, Chowdhury S, Nahas S, et al. Rapid whole-genome sequencing decreases infant morbidity and cost of hospitalization. npj Genomic Medicine. 2018;3(1):10.
Marshall DA, MacDonald KV, Heidenreich S, Hartley T, Bernier FP, Gillespie MK, et al. The value of diagnostic testing for parents of children with rare genetic diseases. Genetics in Medicine. 2019;21(12):2798- 806.
McConkie-Rosell A, Hooper SR, Pena LDM, Schoch K, Spillmann RC, Jiang YH, et al. Psychosocial Profiles of Parents of Children with Undiagnosed Diseases: Managing Well or Just Managing? J Genet Couns. 2018;27(4):935-46.
- Department of Health and Aged Care. Medicare Benefits Schedule health.gov.au: Australian Government 2023 [Available from: https://www9.health.gov.au/mbs/sear ch.cfm?q=73292+&Submit=&sopt=S.
- Department of Health and Aged Care. Medicare Benefits Schedule health.gov.au: Australian Government; 2023 [Available from: http://www9.health.gov.au/mb/fullDis play.cfm?type=itemq=73292&qt=item ].
Clark MM, Stark Z, Farnaes L, Tan TY, White SM, Dimmock D, et al. Meta- analysis of the diagnostic and clinical utility of genome and exome sequencing and chromosomal microarray in children with suspected genetic diseases. npj Genomic Medicine. 2018;3(1):16.
Stark Z, Tan TY, Chong B, Brett GR, Yap P, Walsh M, et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med. 2016;18(11):1090-6.
- Australian Bureau of Statistics. Births, Australia abs.gov.au: ABS; 2022 [Available from: https://www.abs.gov.au/statistics/peo ple/population/births-australia/2021.
Baird PA, Anderson TW, Newcombe HB, Lowry RB. Genetic disorders in children and young adults: a population study. Am J Hum Genet. 1988;42(5):677-93.
Tan NB, Tan TY, Martyn MM, Savarirayan R, Amor DJ, Moody A, et al. Diagnostic and service impact of genomic testing technologies in a neonatal intensive care unit. J Paediatr Child Health. 2019;55(11):1309-14.
Dragojlovic N, van Karnebeek CDM, Ghani A, Genereaux D, Kim E, Birch P, et al. The cost trajectory of the diagnostic care pathway for children with suspected genetic disorders. Genetics in Medicine. 2020;22(2):292- 300.
Fair Work Ombudsman.
Spencer-Tansley R, Meade N, Ali F, Simpson A, Hunter A. Mental health care for rare disease in the UK - recommendations from a quantitative survey and multi-stakeholder workshop. BMC Health Serv Res. 2022;22(1):648.
- Services Australia. Mental health care and Medicare my.gov.au: Australian Government 2023 [Available from: https://www.servicesaustralia.gov.au/m ental-health-care-and- medicare?context=60092.
- Department of Health and Aged Care. Medicare Benefits Schedule health.gov.au: Australian Government 2023 [Available from: http://www9.health.gov.au/mb/fullDisp lay.cfm?type=itemq80110&qt=item].
Guild Care. Social Return on Investment of Guild Care Community Services. Report for Guild Care. 2021.
Goranitis I, Best S, Christodoulou J, Boughtwood T, Stark Z. Preferences and values for rapid genomic testing in critically ill infants and children: a discrete choice experiment. Eur J Hum Genet. 2021;29(11):1645-53.
Brett GR, Martyn M, Lynch F, de Silva MG, Ayres S, Gallacher L, et al. Parental experiences of ultrarapid genomic testing for their critically unwell infants and children. Genetics in Medicine. 2020;22(12):1976-85.
Stark Z, Ellard S. Rapid genomic testing for critically ill children: time to become standard of care? European Journal of Human Genetics. 2022;30(2):142-9.
- Australian Bureau of Statistics. Labour Force Status of Families abs.gov.au: ABS; 2022 [Available from: https://www.abs.gov.au/statistics/labou r/employment-and- unemployment/labour-force-status- families/latest-release.
- Reserve Bank of Australia. Exchange Rates rba.gov.au: RBA 2023 [Available from: https://www.rba.gov.au/statistics/frequ ency/exchange-rates.html.
- Reserve Bank of Australia. Inflation Calculator rba.gov.au: RBA; 2022 [Available from: https://www.rba.gov.au/calculator/an nualDecimal.html.
Cernat A, Hayeems RZ, Ungar WJ. Cascade health service use in family members following genetic testing in children: a scoping literature review. Eur J Hum Genet. 2021;29(11):1601-10.
- Monash IVF. Genetic Carrier Screening Test monashivf.com: Monash IVF 2023 [Available from: https://monashivf.com/services/genet ic-testing/genetic-carrier-screening- kit/.
Saunders CJ, Miller NA, Soden SE, Dinwiddie DL, Noll A, Alnadi NA, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. 2012;4(154):154ra35.
Australian Government Department of the Prime Minister and Cabinet. Best Practice Regulation Guidance Note Value of statistical life2022.
World Health Organisation. Coronavirus disease (Covid-19) who.int: WHO; 2023
- Department of Health and Aged Care. Coronavirus (COVID-19) health.gov.au: Australian Government 2023 [Available from: https://www.aihw.gov.au/reports- data/myhospitals/intersection/activity /apc.
Australian Institute of Health and Welfare. Australian Burden of Disease Study Impact and causes of illness and death in Aboriginal and Torres Strait Islander people 2018. aihw.gov.au: AIHW; 2022.
Basseal JM, Bennett CM, Collignon P, Currie BJ, Durrheim DN, Leask J, et al. Key lessons from the COVID-19 public health response in Australia. The Lancet Regional Health – Western Pacific. 2023;30.
Zhao Y, Leach LS, Walsh E, Batterham PJ, Calear AL, Phillips C, et al. COVID- 19 and mental health in Australia – a scoping review. BMC Public Health. 2022;22(1):1200.
Griffiths D, Sheehan L, van Vreden C, Petrie D, Whiteford P, Sim MR, et al. Changes in work and health of Australians during the COVID-19 pandemic: a longitudinal cohort study. BMC Public Health. 2022;22(1):487.
Department of Health and Aged Care. Evaluation of COVID-19 point-of-care testing in remote and First Nations communities. health.gov.au; 2022.
Parupudi T, Panchagnula N, Muthukumar S, Prasad S. Evidence- based point-of-care technology development during the COVID-19 pandemic. Biotechniques. 2021;70(1):58-67.
Nous. Evaluation of COVID-19 point-of- care testing in remote and First Nations communities. 2022.
Hengel B, Causer L, Matthews S, Smith K, Andrewartha K, Badman S, et al. A decentralised point-of-care testing model to address inequities in the COVID-19 response. Lancet Infect Dis. 2021;21(7):e183-e90.
- The Kirby Institute. Point-of-care COVID-19 testing in remote First Nations communities2023 09/11/23. Available from: https://www.kirby.unsw.edu.au/news/p oint-care-covid-19-testing-remote-first- nations-communities-blueprint- collaborative-health.
- World Health Organisation. Heaptitis C who.int: WHO; 2023 [Available from: https://www.who.int/news-room/fact- sheets/detail/hepatitis-c.
- Hepatitis Australia. Hepatitis C hepatitisaustralia.com: Hepatitis Australia 2023 [Available from: https://www.hepatitisaustralia.com/pa ges/category/hepatitis-c.
Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia Annual surveillance report 2022. kirby.unsw.edu.au: Kirby; 2022.
- Kirby Institute. Monitoring hepatitis C treatment uptake in Australia2022 26/07/2023. Available from: https://www.kirby.unsw.edu.au/researc h/reports/monitoring-hepatitis-c- treatment-uptake-australia-issue-12- july-2022
Grebely J, Markus C, Causer LM, Silk D, Comben S, Lloyd AR, et al. A national programme to scale-up decentralised hepatitis C point-of- care testing and treatment in Australia. The Lancet Gastroenterology & Hepatology. 2023;8(3):204-7.
Scott DN, Palmer MA, Tidhar MT, Stoove PM, Sacks-Davis DRS, Doyle AJS, et al. Assessment of the cost- effectiveness of Australia's risk- sharing agreement for direct-acting antiviral treatments for hepatitis C: a modelling study. Lancet Reg Health West Pac. 2022;18:100316.
Catlett B, Hajarizadeh B, Cunningham E, Wolfson-Stofko B, Wheeler A, Khandaker-Hussain B, et al. Diagnostic Accuracy of Assays Using Point-of-Care Testing or Dried Blood Spot Samples for the Determination of Hepatitis C Virus RNA: A Systematic Review. J Infect Dis. 2022;226(6):1005-21.
Grebely J, Gilliver R, McNaughton T, Conway A, Cunningham E, Henderson C, et al. Single-visit hepatitis C point-of-care testing, linkage to nursing care, and peer- supported treatment among people with recent injecting drug use at a peer-led needle and syringe program: The TEMPO Pilot Study. Int J Drug Policy. 2023;114:103982.
Macisaac M, Whitton B, Anderson J, Hornung M, Cogger S, Elmore K, et al. Rapid point of care HCV testing allows high throughout HCV screening and rapid treatment uptake among PWID attending a medically supervised injecting room. International Conference on Hepatitis Care in Substance Users; Oct 13–15, 2021. 2021.
Conway A, Read P, Gilliver R, McNaughton T, Valerio H, Cunningham EB, et al. Awareness of HCV Status and Preferences for Testing and Treatment among People with Recent Injecting Drug Use at a Peer-Led Needle and Syringe Program: The TEMPO Pilot Study. Viruses. 2022;14(11). The TEMPO Pilot Study. Viruses. 2022;14(11).
- World Health Organization. 2023. Hepatitis C. Accessed from https://www.who.int/news-room/fact- sheets/detail/hepatitis-c.
- Bamford L. From chronic to cured: could Australia be the first country in the world to eliminate hep C?2017 31/10/23. Available from: https://www.kirby.unsw.edu.au/news/c hronic-cured-could-australia-be-first- country-world-eliminate-hep-c.
Singhi P. Central Nervous System Infections in Children: An Ongoing Challenge! The Indian Journal of Pediatrics. 2019;86(1):49-51.
O'Brien MP, Francis JR, Marr IM, Baird RW. Impact of Cerebrospinal Fluid Multiplex Assay on Diagnosis and Outcomes of Central Nervous System Infections in Children: A Before and After Cohort Study. Pediatr Infect Dis J. 2018;37(9):868-71.
- Australian Government Department of Heatlh and Aged Care. National Notifiable Disease Surveillance System 2023 [cited 2023 28 August]. Available from: https://nindss.health.gov.au/pbi- dashboard/.
Huppatz C, Durrheim DN, Levi C, Dalton C, Williams D, Clements MS, et al. Etiology of encephalitis in Australia, 1990-2007. Emerg Infect Dis. 2009;15(9):1359-65.
Sahu RN, Kumar R, Mahapatra AK. Central nervous system infection in the pediatric population. J Pediatr Neurosci. 2009;4(1):20-4.
Gora H, Smith S, Wilson I, Preston- Thomas A, Ramsamy N, Hanson J. The epidemiology and outcomes of central nervous system infections in Far North Queensland, tropical Australia; 2000- 2019. PLOS ONE. 2022;17(3):e0265410.
Disse SC, Zapf A, Schneble F, Fiedler A, Hossain H, von Meyer A. The clinical impact of multiplex PCR panel diagnostics in paediatric meningitis/ encephalitis: a bicenter cohort study. Infection. 2022;50(5):1329-48.
- Tesini BL. Viral Central Nervous System Infections in Children: MSD; 2023 [cited 2023 8 August]. Available from: https://www.msdmanuals.com/en- au/home/children-s-health- issues/common-viral-infections-in- infants-and-children/viral-central- nervous-system-infections-in- children.
- OECD. Stemming the superbug tide in Australia, 2023 [cited 2023 August 25]. Available from: https://www.oecd.org/australia/Stem ming-the-Superbug-Tide-in- Australia.pdf.
IHACPA. National Efficient Price Determination 2023-24. 2023.
- IHACPA. National Hospital Cost Data Collection: IHACPA; 2023 [Available from: https://www.ihacpa.gov.au/health- care/costing/national-hospital-cost- data-collection.
Evans M, Merkel KG, Harder J, Rose DT. Impact of the implementation of a rapid meningitis/encephalitis multiplex polymerase chain reaction panel on IV acyclovir duration: multicenter, retrospective cohort of adult and pediatric patients. Diagnostic Microbiology and Infectious Disease. 2020;96(2):114935.
Beaman MH. Community-acquired acute meningitis and encephalitis: a narrative review. Med J Aust. 2018;209(10):449-54.
Chong BSW, Kennedy KJ. Comparison of a commercial real- time PCR panel to routine laboratory methods for the diagnosis of meningitis-encephalitis. Pathology. 2021;53(5):635-8.
- National Blood Authority. Patient Blood Management Canberra: National Blood Authority (Australia); [cited 2023 27 July]. Available from: https://www.blood.gov.au/patient- blood-management-pbm.
Shen L, Tabaie S, Ivascu N. Viscoelastic testing inside and beyond the operating room. J Thorac Dis. 2017;9(Suppl 4):S299-s308.
- Blood Synergy. Making better use of blood 2023 [cited 2023 August 15]. Available from: https://bloodsynergy.org/.
- National Health and Medical Research Council (NHMRC). Addressing Australia’s national transfusion research priorities 2021 [Available from: https://www.nhmrc.gov.au/about- us/news-centre/addressing-australias- national-transfusion-research- priorities.
University of Melbourne. Saving lives wih less blood 2023 [Available from: .
National Blood Authority. Patient Blood Management Guidelines: Module 2 Perioperative. blood.gov.au; 2012.
- World Health Organization (WHO). The urgent need to implement patient blood management: policy brief 2021 [cited 2023 16 August]. Available from: https://www.who.int/publications/i/ite m/9789240035744.
Nath SS, Pandey CK, Kumar S. Clinical application of viscoelastic point-of-care tests of coagulation-shifting paradigms. Ann Card Anaesth. 2022;25(1):1-10.
Leahy MF, Hofmann A, Towler S, Trentino KM, Burrows SA, Swain SG, et al. Improved outcomes and reduced costs associated with a health-system- wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57(6):1347- 58.
- The Hon Mark Butler MP. Minister for Health and Aged Care – Speech – National Press Club – 2 May 20232023 31/10/23. Available from: https://www.health.gov.au/ministers/th e-hon-mark-butler- mp/media/minister-for-health-and- aged-care-speech-national-press-club- 2-may-2023.
Pathology Technology Australia MC. Diagnostic Technology Sovereign Capability & Resilience - A National Action Plan pathologytechnology.org.au: Pathology Technology Australia 2023 31/10/23..
Saravanan KA, Panigrahi M, Kumar H, Rajawat D, Nayak SS, Bhushan B, et al. Role of genomics in combating COVID-19 pandemic. Gene. 2022;823:146387.
- Australian Government Department of Health and Aged Care. 1476 - Genetic testing for childhood syndromes msac.gov.au: Australian Government 2021 [Available from: http://www.msac.gov.au/internet/msa c/publishing.nsf/Content/1476- public#:~:text=Application%20Detail& text=A%20phenotype%2Ddriven%20li st%20of,in%20the%20affected%20ind ividual%27s%20condition].
Mattick JS, Dinger M, Schonrock N, Cowley M. Whole genome sequencing provides better diagnostic yield and future value than whole exome sequencing. Medical Journal of Australia. 2018;209(5):197-9.
- Australian Government Department of Health and Aged Care. MBS Online Medicare Benefits Schedule- May 2020 News mbsonline.gov.au: Australian Government 2020 [Available from: https://www.mbsonline.gov.au/intern et/mbsonline/publishing.nsf/Content/ 20200501-News].
Kim MJ, Yum MS, Seo GH, Lee Y, Jang HN, Ko TS, et al. Clinical Application of Whole Exome Sequencing to Identify Rare but Remediable Neurologic Disorders. J Clin Med. 2020;9(11).
Horton RH, Lucassen AM. Recent developments in genetic/genomic medicine. Clin Sci (Lond). 2019;133(5):697-708.
- Genetic Alliance; District of Columbia Department of Health. Chapter 2, Diagnosis of a Genetic Disease 2010 16/11/23. In: Understanding Genetics: A District of Columbia Guide for Patients and Health Professionals [Internet]. Available from: https://www.ncbi.nlm.nih.gov/books/N BK132142/.
Mumford V, Baysari MT, Kalinin D, Raban MZ, McCullagh C, Karnon J, et al. Measuring the financial and productivity burden of paediatric hospitalisation on the wider family network. J Paediatr Child Health. 2018;54(9):987-96
Aldridge CE, Osiovich H, Siden H, Study R, Study G, Elliott AM. Rapid genome- wide sequencing in a neonatal intensive care unit: A retrospective qualitative exploration of parental experiences. Journal of Genetic Counseling. 2021;30(2):616-29.